Kymriah: Uses, Side Effects, and Interactions

- Kymriah
- 01 Sep 2023
Overview
Overview
Tisagenlecleucel (Kymriah) injection is employed to cure specific acute lymphoblastic leukemia (ALL- acute lymphatic/ lymphoblastic leukemia) in patients who are younger than 25 years of age and whose condition has recurred or whose bodies do not react to other treatment or treatments.

Uses
What is Kymriah used for?
- It is also used to treat a specific form of non-Hodgkin’s lymphoma, which is a form of cancer that initiates in a variety of white blood cells that are normally responsible for fighting infection. 1Uses | Researched based study from MedlinePlus
- It is further employed for treating follicular lymphoma, which is a form of cancer that begins in the white blood cells and grows slowly, in adults when treatment with at least two other medications has failed to control the disease or the disease has returned.
Mode of Action
How does Kymriah perform its action?
- Tisagenlecleucel injection is a sort of autologous cellular immunotherapy, which is a class of treatment that is made by extracting cells from the patient’s own blood rather than utilizing cells from other sources. 2Mode of Action | Researched based study from European Medicines Agency
- It does this by activating the immune system, which is a collection of tissues, and organs that defend the body from the action of bacteria, viruses, cancerous cells, and other things that lead to diseases.
- This causes the immune system to fight cancer cells, which is its primary mechanism of action.
Side Effects
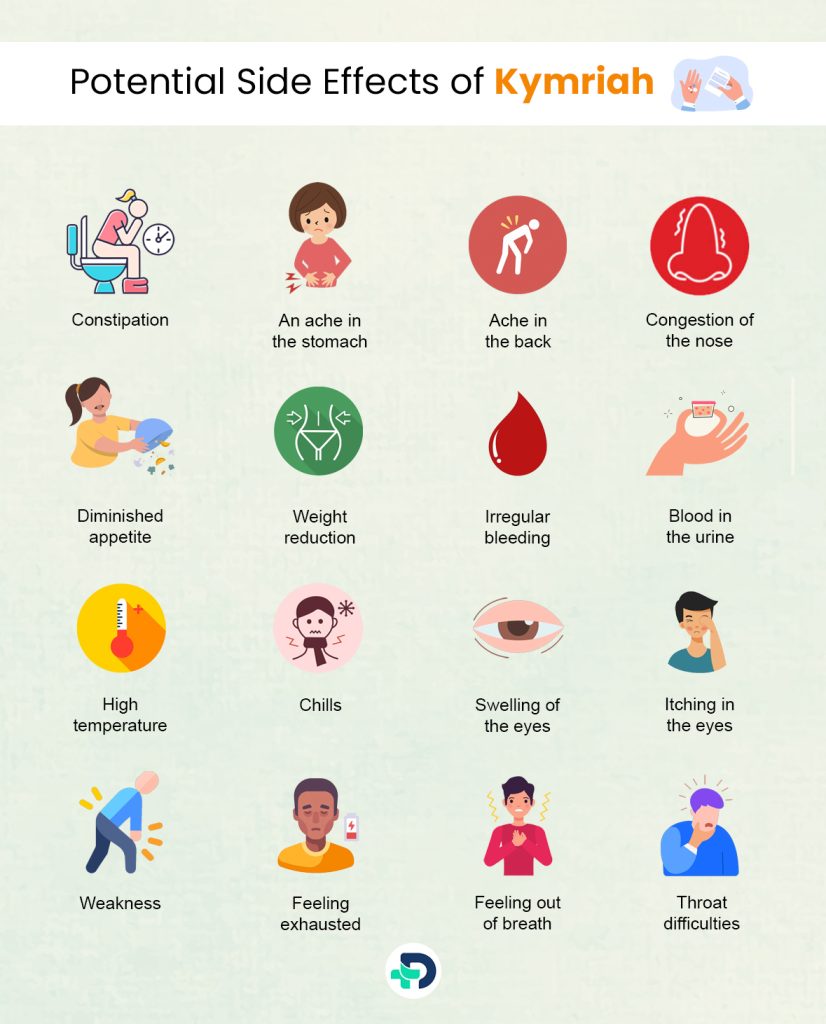
Potential Side Effects of Kymriah
The injection of Tisagenlecleucel might potentially induce adverse consequences. Inform your physician if any of the following symptoms persist for an extended period of time or are particularly severe: 1Side Effects | Researched based study from MedlinePlus
- Constipation
- An ache in the stomach
- Ache in the back
- Congestion of the nose
- Diminished appetite
- Weight reduction
Some of the adverse effects may be rather significant. Call your physician right once or go to the nearest hospital emergency room if you develop any of the following symptoms, or any of the symptoms indicated in the section labeled “IMPORTANT WARNING”: 1Side Effects | Researched based study from MedlinePlus
- Irregular bleeding or bruising
- Blood in the urine
- Bowel motions that are red or black like tar
- Vomiting blood or material that resembles coffee grounds
- High temperature
- Chills
- Decreased frequency or amount of urination
- Swelling of the eyes, face, lips, tongue, throat, arms, hands, feet, ankles, or lower legs
- A rash
- Itching in the eyes
- Weakness
- Feeling exhausted
- Feeling out of breath
- Swelling of the Eyes, Lips, Tongue, and Neck
- Throat difficulties like swallowing and itching
It is possible that getting an injection of Kymriah will make your chance of acquiring some cancers higher. Have a consultation with your healthcare expert about the possible side effects of using Kymriah before receiving treatment.
The majority of people who use Kymriah report experiencing substantial adverse effects, including: 3Side Effects | Researched based study from National Library of Medicine
- Cytokine release syndrome (CRS)
- Neurological toxicities
- Hemophagocytic lymphohistiocytosis /macrophage activation syndrome (HLH/MAS)
Patients and caregivers are given detailed instructions to keep an eye out for signs and symptoms associated with both CRS and neurological toxicities, and the health care practitioner is responsible for treating both of these disorders.
Cytokine release syndrome (CRS) 3Side Effects | Researched based study from National Library of Medicine
- Cytokines are chemicals that are involved in inflammation and can impact the operation of numerous organs.
- CRS is caused by a broad release of these cytokines, which causes inflammation.
- Cytokines might be produced either by the T cells induced by Kymriah or by different immune cells found in the body of the patient.
- A high temperature, chills, trouble breathing, intense pain in the muscles or joints, extreme nausea, puking, diarrhea, extremely low blood pressure, and feeling faint or lightheaded are all symptoms of CRS.
- CRS manifests itself anywhere between one and ten days following a Kymriah infusion, and the patient should immediately inform their healthcare physician if they have any symptoms of the condition.
Toxicity to the nervous system 3Side Effects | Researched based study from National Library of Medicine
- Some of the cytokines that are generated during CRS might result in the blood-brain barrier being disrupted, which can contribute to the development of neurological toxicities.
- Confusion, altered or diminished awareness, seizures, loss of balance, trouble speaking and comprehending, and loss of control over one’s body are some of the symptoms of neurological toxicities.
- Neurological toxicities often manifest themselves roughly seven days after a Kymriah infusion, although they might manifest themselves eight weeks or later after the infusion.
Hemophagocytic lymphohistiocytosis Macrophage activation syndrome (HLH/MAS)
- A number of cytokines that are produced as a result of cytokine release syndrome might lead to unrestrained triggering of the immune system, which can cause HLH/MAS.
- In both HLH and MAS, immune cells including lymphocytes and macrophages begin acting on and killing healthy tissues, which results in extensive harm to the tissues and inflammation.
- Low blood pressure, low oxygen levels in the blood, changed organ function, and multiple organ failure are some of the symptoms of HLH/MAS, which commonly appear anywhere from three days to eighteen days after receiving Kymriah.
Administration
How should you take this medication exactly?
- Tisagenlecleucel injection is administered intravenously (into a vein) by a physician or nurse at a physician’s office or an infusion facility.
- The medication is administered as a suspension, which is a liquid.
- A single dose of it is often administered over a period of time ranging from up to sixty minutes.
- In order to get your body ready for the Tisagenlecleucel treatment, your physician or nurse will give you other chemotherapy drugs first. This will facilitate the body to react better to the action of Tisagenlecleucel. 4Administration | Researched based study from Understanding Cancer Immunotherapy Research
- A specimen of your WBCs will be obtained using a technique called leukapheresis, which is a process that extracts white blood cells out of the body.
- This treatment will take place around three to four weeks before your dosage of Tisagenlecleucel injection is scheduled to be administered.
- This process will take around three to six hours to complete and may have to be carried out more than once.
- Because of the fact that this treatment is created from your own cells, it can only be administered to you.
- It is essential that you keep all of your planned cell collection appointments and that you do not miss any of your treatment doses, thus it is critical that you arrive on time.
- For a period of at least four weeks from receiving your dosage of Tisagenlecleucel, you should make it a point to remain within a radius of two hours of the facility at which you had the therapy.
Your healthcare practitioner will check to determine if the therapy is effective and will monitor you for any potential adverse reactions that may occur. Have a discussion with your physician about how to be ready for the leukapheresis treatment and understand what you need to expect during and post-procedure.
Warnings
Aspects to remember before using Kymriah 3Warnings | Researched based study from National Library of Medicine 4Warnings | Researched based study from Understanding Cancer Immunotherapy Research
REMS Program 4Warnings | Researched based study from Understanding Cancer Immunotherapy Research
- Due to the possibility of major adverse effects, Kymriah is presently only available via a Risk Evaluation and Mitigation Strategy (REMS) program.
- This limitation exists because of the potential for the drug to cause these adverse effects.
- Healthcare institutions that intend to treat patients with Kymriah are required to first qualify for the program and assure effective monitoring and treatment of adverse effects related with the therapy before they can begin treating patients with the medication.
Age 4Warnings | Researched based study from Understanding Cancer Immunotherapy Research
- It has not been determined whether or if Kymriah is both safe and effective for individuals with acute lymphoblastic leukemia who are under the age of 2 or who are above the age of 65.
- Patients younger than 18 years old who have diffused large B-cell lymphoma or follicular lymphoma have not been studied sufficiently to draw conclusions on the effectiveness or safety of Kymriah.
Patients suffering from certain disorders
- Patients who are suffering from active hepatitis B virus (HBV), HCV, or human immunodeficiency virus (HIV) are not eligible to receive Kymriah at this time due to the product’s exclusionary nature.
- Kymriah is not authorized for use as a therapy for individuals who have primary central nervous system lymphoma. individuals with central nervous system lymphoma.
Precautions for women
- Concerning fertility, pregnancy, and breastfeeding, it is unknown what effects Kymriah has on either male or female fertility.
- Women who have conceived or want to conceive in the future should not take Kymriah.
- It is not known whether or not using Kymriah during nursing has any problems, but it is also not possible to rule out the possibility of such dangers.
Vaccination with live virus
- Vaccination using live viruses (such as measles/mumps/rubella [MMR]) is not advised for a minimum of six weeks before the beginning of lymphodepleting chemotherapy, at the time of therapy with Kymriah, and until the immune cell counts return to normal levels after therapy with Kymriah. 4Warnings | Researched based study from Understanding Cancer Immunotherapy Research
- This is because live virus vaccines can cause a patient’s immune system to become compromised.
Precautions
What precautionary actions should one take while consuming Kymriah?
- It is imperative to notify your healthcare provider and pharmacist of any allergies you may have to tisagenlecleucel, other medications, dimethyl sulfoxide (DMSO), dextran 40, or any other components present in the tisagenlecleucel injection prior to receiving the injection. 1Precautions | Researched based study from MedlinePlus
- It is advisable to consult with both your healthcare provider and pharmacist regarding any supplementary prescribed or over-the-counter medicines, vitamins, dietary supplements, or herbal medications that you are currently consuming or are planning to take later. It is conceivable that the physician may need to modify the dosages of your medications or closely monitor your condition to ascertain the presence of any adverse effects.
- It is imperative to inform your healthcare provider of any previous adverse reactions to prior chemotherapy interventions, such as respiratory difficulties or cardiac arrhythmias. It is ideal to put these issues across to your healthcare provider.
- Furthermore, it is imperative to disclose to your healthcare provider any history of hepatitis B or C, viral infections that target the liver and can result in severe liver damage, human immunodeficiency virus (HIV) infection, as well as any preexisting lung, kidney, heart, or liver conditions. 1Precautions | Researched based study from MedlinePlus
- It is important to note that the administration of tisagenlecleucel may result in somnolence, as well as cognitive impairment, asthenia, vertigo, and perhaps epileptic episodes in certain individuals. It is recommended to observe a minimum waiting period of 8 weeks following the administration of tisagenlecleucel before engaging in activities such as operating a motor vehicle or utilizing machinery.
- Following the administration of tisagenlecleucel, it is advised to refrain from donating blood, organs, tissues, sperm, or cells for transplantation.
- It is advisable to see a healthcare professional in order to evaluate the necessity of any vaccinations. Prior to initiating chemotherapy, during tisagenlecleucel treatment, and until medical clearance is given indicating immune system recovery, it is imperative to seek professional guidance before receiving any vaccinations for a minimum duration of six weeks.
- A patient who has just received Kymriah should wait at least 8 weeks before driving, engaging in potentially dangerous vocations or activities, or operating heavy machinery due to the drug’s potential effects on their ability to do so.
- Patients should also avoid using alcohol.
Interactions
Interactions with Kymriah
No pharmacokinetic or pharmacodynamic studies of how Kymriah interacts with other drugs have been done on children or adults. Agents that are known to stop T-cells from working have not been studied together. Low-dose steroids given as part of the treatment plan for cytokine release syndrome do not affect the growth and survival of CAR-T cells. Agents that are known to make T-cells work better have not been tested together, so the benefits are unknown. 5Interactions | Researched based study from European Medicines Agency
Live vaccines: 5Interactions | Researched based study from European Medicines Agency
No research has been done on the safety of getting live vaccines while taking Kymriah or after. As a safety measure, live vaccines shouldn’t be given at least 6 weeks before lymphodepleting chemotherapy starts, during Kymriah treatment, or until the immune system has fully recovered after treatment.
Interference with HIV infection testing in the laboratory
Patients who have been treated with Kymriah may get false-positive findings with certain HIV lab tests due to the medication.
Any feedback on this article?
 This Articles content was accurate
This Articles content was accurate Very Informative Article
Very Informative Article I have a question or a comment
I have a question or a comment
 This article contains inaccurate content
This article contains inaccurate content This article was not helpful
This article was not helpful I have a question or a comment
I have a question or a comment
We appreciate your helpful feedback!
Checkout our social pages
References
-
MedlinePlus
Uses | Precautions | Side Effects
-
European Medicines Agency
Mode of action
-
National Library of Medicine
Warning | Side Effects
-
Understanding Cancer Immunotherapy Research
Administration | Warnings
-
European Medicines Agency
Interactions