Mounjaro: Uses, Side Effects and Precautions

- Mounjaro
- 06 Sep 2023
Introduction
Introduction
For the treatment of type 2 diabetes, a drug called Mounjaro is used. Tirzepatide is one of the active ingredients. We shall examine the numerous facets of this medication in this article, including its indications, side effects, dosage, and potential interactions.

Uses
Uses of Mounjaro
Diabetes type-2
- It should be used with a nutritious diet and frequent exercise by adults with type 2 diabetes.
- It is a chronic metabolic disorder in which the body either stops producing enough insulin to maintain blood sugar levels within the normal range or becomes resistant to insulin’s effects. As blood glucose levels rise, a number of health issues could develop over time.
- Tirzepatide is the primary component of this medication.
Limitations on use
- It cannot be used by those with type 1 diabetes mellitus.
- For those who have experienced pancreatitis, it might not be safe. 1Uses | Researched based study from National Institutes of Health
Side Effects
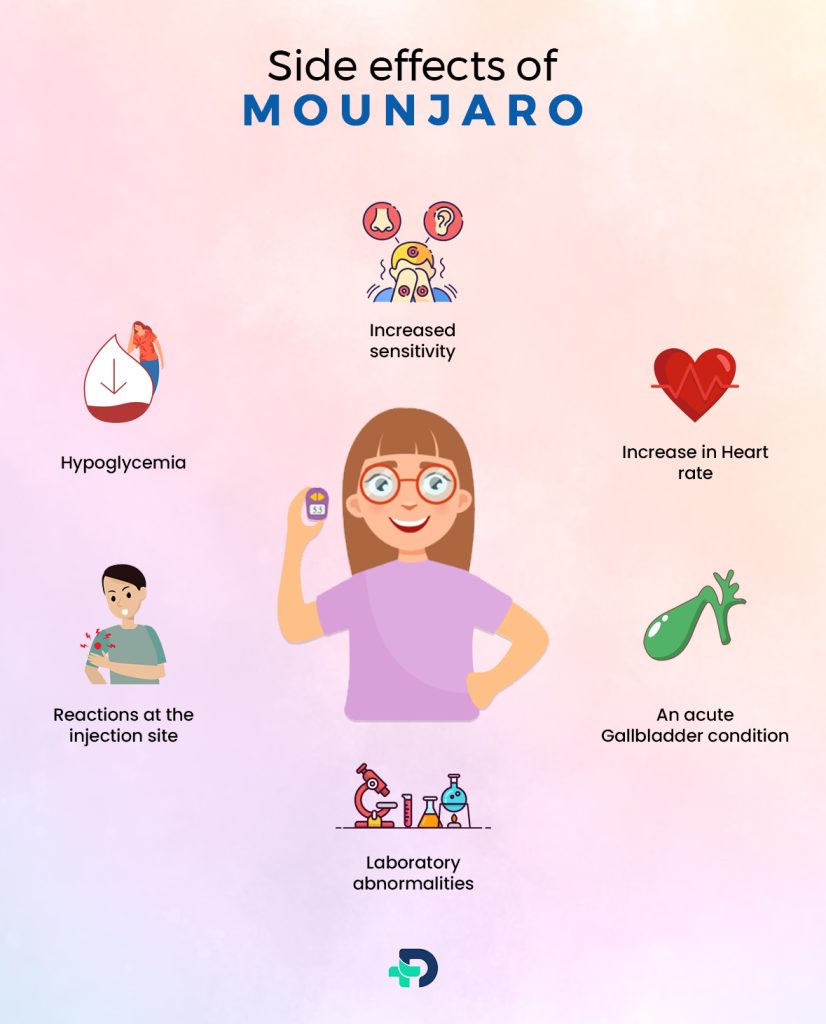
Side Effects
Common adverse effects
- Vomiting
- Constipation
- Low appetite
- Diarrhea, and other symptoms.
It typically happens during dose increase and becomes more negligible over time.
Hypoglycemia
Depending on the dose and treatment combination, it occurred at varied rates. when sulfonylurea is also used, more regularly. Low blood sugar may result in.
- Shakiness
- Jitteriness
- Anxiety
- Perspiration
- Hunger
- Weakness
- Fast heartbeat
- Headache
- Dizziness.
Increase in Heart rate
- The heart rate is elevated as a result of it. Higher dose groups reported more significant instances of sinus tachycardia.
Increased sensitivity
- Eczema and urticarial reactions due to hypersensitivity have been seen.
Reactions at the injection site
- Painful reactions at the injection site are possible. Patients who have anti-Tirzepatide antibodies experience this more frequently.
An acute Gallbladder condition
- This has been reported in just a few of the patients. If you experience stomach pain, skin or eye yellowing, fever, or clay-colored feces, call your doctor right away.
Laboratory abnormalities
- Amylase and lipase concentrations in the serum from the pancreas may rise.
- Without other pancreatic symptoms, the clinical relevance is questionable. 2Side Effects | Researched based study from Food and Drug Administration
Dosage
Instructions for Dosage
- Available in single-dosage pre-filled pens, the medication is a clear, slightly yellow solution.
- It is available in a variety of strengths and volumes.
- Start your medication with a 2.5 mg subcutaneous injection per week, not for glycemic control.
- After four weeks, increase the dosage to 5 mg once each week.
- Whenever more control is required, up the dose by 2.5 mg every four weeks.
- The maximum dose is 15 mg administered once per week. 2Dosage | Researched based study from Food and Drug Administration
- If a dose is missed, take it within four days. After four days, omit the missed dose and take it once a week.
- At least three days must pass between dosages, so change the weekly day.
Administration
Essential Rules for the Administration
- Apply it subcutaneously to the upper arm, thigh, or abdomen.
- Alternate injection sites
- Visually check for clarity; if there are any specks or discoloration, do not use them.
- Co-administration when utilizing insulin injections separately is acceptable, but not adjacent injections. 2Administration | Researched based study from Food and Drug Administration
Overdose
Overdose
- Contact poison control for the most recent guidance if an overdose occurs. Depending on the symptoms, start the appropriate supportive care.
- Since Tirzepatide has a half-life of about five days, it could be required to monitor and treat the symptoms for a while. 2Overdose | Researched based study from Food and Drug Administration
Storage
Storage
- Please keep it in a refrigerator between 2 and 8 degrees Celsius.
- Single-dose pens can be kept for up to 21 days below 30 degrees Celsius.
- It shouldn’t be frozen; don’t use it if it is.
- To protect it from light, keep it in its original carton. 2Storage | Researched based study from Food and Drug Administration
Precautions
Warnings and Precautions
Thyroid C-cell tumor risk
- When exposed to clinically significant levels, Tirzepatide produced thyroid sea cell tumors in rats.
- If this medication results in these tumors in humans is unknown.
- Patients with multiple endocrine neoplasia syndrome or those with a history of thyroid sea cell tumors should not undergo it.
Risk of Pancreatitis
- GLP-1 agonists, such as Mounjaro, can cause acute pancreatitis when used.
- If any symptoms are thought to exist, the medication should be stopped.
Low blood sugar with Insulin use
- The risk of hypoglycemia may increase when this medication is taken with insulin or insulin secretagogues.
- Adjust dosages to reduce risk and teach patients hypoglycemic symptoms.
Increased sensitivity
- With this medication, some people may suffer severe hypersensitivity responses, including a severe rash, rapid heartbeat, swelling of the face, lips, tongue, or throat, and breathing or swallowing difficulties.
- If necessary, the medication should be stopped.
- Patients with a history of these responses to GLP-1 agonists should use caution.
Acute kidney damage
- Due to GI issues, it may cause dehydration, which may result in kidney problems. Weakness, less frequent urination, and confusion are symptoms.
- Patients experiencing GI side symptoms should have their renal function checked, especially if they already have renal problems.
Severe Gastrointestinal disease
- Patients with serious GI issues should avoid it.
Difficulties with Diabetic Retinopathy
- Rapid improvements in glucose regulation could momentarily exacerbate diabetic retinopathy. Contact your doctor if your vision becomes hazy or changes.
An acute Gallbladder condition
- With this drug monitor, some gallbladder problems are reported; if suspicion is raised, an investigation is conducted.
Pregnancy-related issues
- Its usage in expectant mothers is poorly understood, and poorly managed diabetes during pregnancy puts both the mother and the fetus at danger.
- Use only if the benefits outweigh any potential hazards to the fetus before conception.
Lactation
- No information is available regarding the effects of Tirzepatide on babies or its availability in breast milk.
Reproductive potential
- Due to delayed stomach emptying, it might compromise the effectiveness of oral contraceptives.
- Your doctor might advise moving from oral to non-oral contraceptives or adding barrier contraception for the first four weeks after starting the technique and during escalation.
- For renal impairment, Mounjaro does not require a dose change.
- When starting or increasing doses in patients with renal problems, renal function monitoring is advised. 2Precautions | Researched based study from Food and Drug Administration
Interactions
Drug Interactions
Concurrent use of Insulin or Insulin Secretagogues
- It was thought to reduce the insulin dosage or other insulin secretagogues, such as sulfonylureas, when starting the medication.
- By doing this, the chance of hypoglycemia can be reduced.
Effect on Oral Medicines
- It delays stomach emptying, which may interfere with the absorption of oral drugs being taken simultaneously.
- When taking medications orally with Mounjaro, caution is advised.
Maintaining track of Oral meds
- This is crucial for medications with strict concentration requirements or those with limited therapeutic use, like warfarin.
Hormonal Contraceptives
- Four weeks after taking the medication and after each dose increase, people using oral hormonal contraceptives should switch to non-oral methods or add a barrier technique.
- Contraceptives that are not hormonal shouldn’t be impacted. 2Interactions | Researched based study from Food and Drug Administration
The bottom line
The type 2 diabetes drug Mounjaro has potential benefits. However, caution is advised due to side effects that have been noted. Its effects on lactation and pregnancy must be carefully considered. The danger of thyroid cancer should be considered for those who are susceptible. Its use should be approached cautiously, paying attention to potential hazards and unique patient profiles, even though clinical trials have proven promising.
Any feedback on this article?
 This Articles content was accurate
This Articles content was accurate Very Informative Article
Very Informative Article I have a question or a comment
I have a question or a comment
 This article contains inaccurate content
This article contains inaccurate content This article was not helpful
This article was not helpful I have a question or a comment
I have a question or a comment
We appreciate your helpful feedback!
Checkout our social pages
References
-
National Institutes of Health
Uses
-
Food and Drug Administration
Side effects | Dosage | Administration | Overdose | Storage | Precautions | Interactions