Humira: Uses, Dosage, Side effects and Interactions

- Humira
- 22 Aug 2023
Overview
What is Humira?
In medicine, numerous advancements have been made to combat various diseases and improve patient outcomes. One such innovation is the drug Humira, which has transformed the way many chronic inflammatory diseases are treated. Humira is a brand-name prescription of adalimumab, which belongs to a class of drugs called tumor necrosis factor (TNF) inhibitors.1Overview| Researched based study from Fda.gov This article delves into the mechanism of action, uses, dosage, administration, storage, side effects, risks, contraindications, precautions, and interactions associated with Humira.
How does Humira work?
- TNF-alpha (tumor necrosis factor-alpha), a protein that is essential for inducing inflammation, is targeted and blocked by Humira.
- By inhibiting TNF-alpha, Humira effectively reduces inflammation and its associated symptoms.2Overview| Researched based study from Dailymed.nlm.nih.gov
- TNF-alpha is involved in the pathogenesis of rheumatoid arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, psoriatic arthritis, and ulcerative colitis.
Uses
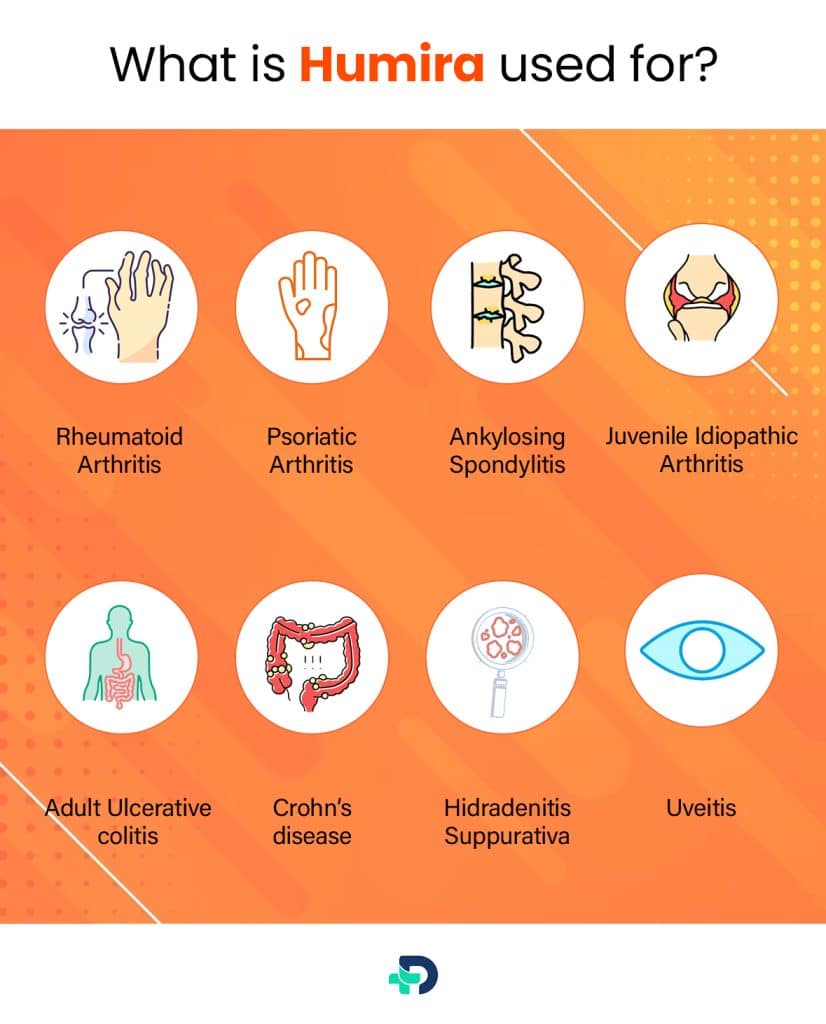
What is Humira used for?
Humira is an FDA-approved drug for the treatment of:
- Rheumatoid Arthritis
- Psoriatic Arthritis
- Juvenile Idiopathic Arthritis
- Ankylosing Spondylitis
- Ulcerative colitis
- Crohn’s disease
- Hidradenitis Suppurativa
- Plaque psoriasis
- Uveitis
Rheumatoid Arthritis (RA)
- Humira benefits adult RA patients with moderate to severe disease activity by easing signs and symptoms, slowing the course of structural harm, and enhancing physical function.1uses| Researched based study from Fda.gov
Psoriatic Arthritis
- A prescription medication called Humira is used either by itself or in combination with other medicines to lessen the symptoms and signs of psoriatic arthritis in adults.1uses| Researched based study from Fda.gov It also helps prevent additional damage to your bones and joints and your capacity to carry out daily tasks.
Juvenile Idiopathic Arthritis
- Even children in whom other non-biologic drugs have not sufficiently benefited may help to lessen the symptoms and signs of moderate to severe polyarticular JIA.1uses| Researched based study from Fda.gov
- Methotrexate or Humira can be combined for treatment.
Ankylosing Spondylitis
- Adult patients on Humira experience fewer symptoms and signs of active ankylosing spondylitis.3Uses| Researched based study from Nlm.nih.gov
Adult Ulcerative colitis
- Adults and children five years and older with moderate to severe ulcerative colitis are treated with the prescription drug Humira.
- Humira’s efficacy has not been proven in patients who no longer respond to or are intolerable to TNF blockers.
Crohn’s disease
- Adults and children aged six and older who have moderate to severe Crohn’s disease (CD) may use the prescription drug Humira.1uses| Researched based study from Fda.gov
Hidradenitis Suppurativa
- Reduce the signs and symptoms of mild to severe hidradenitis suppurativa in individuals 12 and older.
Plaque psoriasis
- Humira treats adults with moderate to severe persistent plaque psoriasis1uses| Researched based study from Fda.gov who are prepared for systemic therapy or phototherapy and monitored by a physician who will determine whether other systemic therapies are more appropriate.
Uveitis
- Humira treats non-infectious pan, posterior, and intermediate (middle) uveitis in adults as well as children two years of age and older.1uses| Researched based study from Fda.gov
Forms
Available forms
Humira is administered via a subcutaneous injection of a liquid solution.
It is available in the dose forms and strengths listed below:
- Single-dose prefilled pen – 40 mg/0.4 ml, 40 mg/0.8 ml, and 80 mg/0.8 ml.
- Single-dose prefilled syringe – 10 mg/0.1 ml, 10 mg/0.2 ml, 20 mg/0.2 ml, 20 mg/0.4 ml, 40 mg/0.4 ml, 40 mg/0.8 ml, and 80 mg/0.8 ml.
- Single-dose vial for use only by healthcare professionals – 40 mg/0.8 ml.1Forms| Researched based study from Fda.gov
Dosage
Administration and Dosage
Humira is typically administered via subcutaneous injection (under the skin). however, the dosage may differ based on the specific health condition, age, and body weight of an individual.
The dosage for each condition may be as follows:
For Ankylosing Spondylitis, Rheumatoid Arthritis, and Psoriatic Arthritis:
- 40 mg once in two weeks.1Dosage| Researched based study from Fda.gov
For Juvenile Idiopathic Arthritis or pediatric uveitis:
Dosage for patients two years of age or older may depend on the body weight as follows:
- 10 to 15 kg – 10 mg once in two weeks.
- 15 kg to 30 kg – 20 mg once in two weeks.
- More than 30 kgs – 40 mg once in two weeks.1Dosage| Researched based study from Fda.gov
For Adult Crohn’s and Ulcerative colitis:
- Initial dose – 160 mg. (Day 1)
- Second dose – 80 mg. (Day 15)
- Maintenance dose – 40 mg once in two weeks. (Begins on day 29)
For Pediatric Crohn’s disease:
Dosage for patients six years or older may depend on body weight.
For children from 17 to 40 kg:
- Initial dose – 80 mg (Day 1)
- Second dose – 40 mg (Day 15)
- Maintenance dose – 20 mg once every two weeks. (Begins on day 29)
For children above 40 kg:
- Initial dose – 160 mg (Day 1)
- Second dose – 80 mg (Day 15)
- Maintenance dose – 40 mg once every two weeks (Begins on Day 29)
For Hidradenitis Suppurativa
Adults and adolescents 12 years or older (weighing above 60 kgs):
- Initial dose – 160 mg (Day 1)
- Second dose – 80 mg (Day 15)
- Maintenance dose – 40 mg every week (Begins on Day 29)
Adolescents 12 years and older (weighing 30 to 60 kgs):
- Initial dose – 80 mg (Day 1)
- Second dose – 40 mg (Day 8)
- Maintenance dose – 40 mg once in two weeks.
For Plaque psoriasis or adult uveitis
- Initial dose – 80 mg.
- Maintenance dose – 40 mg once in two weeks (starting one week after the initial dose.)1Dosage| Researched based study from Fda.gov
The amount and frequency of administration may be adjusted dependent on the patient’s reaction to treatment.
Side effects
Side effects of Humira
Humira may result in specific side effects, which could include:
- Injection site reactions – redness, swelling, and pain
- Upper respiratory tract infections like sinusitis.1Side effects| Researched based study from Fda.gov
- Headache
- Skin rashes
Precautions
Risks and precautions
- Hypersensitivity – some individuals may be allergic to Humira or its content and develop severe allergic reactions. If you develop any allergic symptoms like swelling of the face, lips, throat, or tongue, difficulty breathing, or itching with rashes after taking this, get immediate medical help.
- Severe infection – FDA has given a box warning on the drug as taking Humira may increase a patient’s risk of developing severe infections like tuberculosis, pneumonia, sepsis, etc. Humira must not be used when a condition is present. If a disease appears, keep a close eye on it and discontinue using Humira if it worsens.4Side effects| Researched based study from Medsafe.govt.nz
- Risk of developing cancer – cancers like lymphoma have developed in individuals on Humira. Hence, FDA has issued a boxed warning on the drug for healthcare professionals and patients to be aware of while using it.
- Invasive fungal infections – Patients who get a systemic illness while taking Humira should think about receiving empiric antifungal medication if they live in or frequently visit areas where fungal infections like mycosis are common.
- Hepatitis B virus reactivation – Keep track of HBV carriers during and after treatment. Stop taking Humira if reactivation occurs and start antiviral therapy.
- Heart failure – Humira can worsen congestive heart failure or develop a new one. When administering Humira to heart failure individuals, use caution and keep close tabs on them.
- Pregnant or nursing – Inform your physician if you are nursing a baby, intend to get pregnant, or are already pregnant. Call your doctor right away if you find out you’re pregnant while taking adalimumab injectable products.5Precautions| Researched based study from Medlineplus.gov
- Blood-related issues – Red blood cell, white blood cell, or platelet levels may drop as a result of Humira usage.
- Neurological problems – TNF inhibitors, such as Humira, have occasionally been linked to the start or worsening of peripheral and central demyelinating illnesses, such as multiple sclerosis (MS), optic neuritis, and Guillain-Barré syndrome.2Precautions| Researched based study from Dailymed.nlm.nih.gov
Interactions
Interactions
Humira may interact with certain medications, and disclosing all medications taken to healthcare providers is essential.
- Abatacept – When used with Humira, abatacept may raise the risk of life-threatening infections1Interactions| Researched based study from Fda.gov
- Anakinra – When used with Humira, Anakinra may raise the risk of life-serious infections. 1Interactions| Researched based study from Fda.gov
- Live vaccines – avoid live vaccines in individuals who are treated with Humira. Be sure to have completed all vaccines before starting with Humira.1Interactions| Researched based study from Fda.gov
Humira might interact with other medicines, changing how well they work or raising the chance of adverse effects. Before taking Humira, patients should tell their physician about all the medications they’re currently taking, including prescription, over-the-counter, and herbal supplements.
Any feedback on this article?
 This Articles content was accurate
This Articles content was accurate Very Informative Article
Very Informative Article I have a question or a comment
I have a question or a comment
 This article contains inaccurate content
This article contains inaccurate content This article was not helpful
This article was not helpful I have a question or a comment
I have a question or a comment
We appreciate your helpful feedback!
Checkout our social pages
References
-
FOOD AND DRUG ADMINISTRATION
Humira (adalimumab) label | Overview | Uses | Dosage | Forms
-
DailyMed
HUMIRA- adalimumab kit | Overview
-
National Library of Medicine
Efficacy and safety of adalimumab in ankylosing spondylitis | Uses
-
Medsafe
Humira | Precautions
-
Medline Plus
Adalimumab Injection | Precautions