Eysuvis: Uses, Side effects, Precautions and Interactions

- Eysuvis
- 22 Aug 2023
Overview
What is Eysuvis?
Eysuvis is the first sole corticosteroid FDA-approved medication designed specifically for the short term treatment (highest duration of two weeks) of the dry eye disease.
Approximately 38 million individuals in the United States 1Overview| Researched based study from Nlm.nih.gov exhibit symptoms of dry eye disease, yet only 17.2 million have received an official diagnosis. Common risk factors for various conditions often include being female, postmenopausal, and advanced age.
The treatment of DED should focus on the cause rather than focusing only on symptom management. Inflammation causes an unstable tear film, it disrupts homeostasis, induces stress on the ocular surface, damages the ocular surface and ultimately leads to tissue breakdown.
About Eysuvis
- Eysuvis is classified as an ophthalmic steroid. The generic name given to Eysuvis is Loteprednol etabonate. This formulation is an ophthalmic suspension containing benzalkonium chloride. Since Eysuvis it’s a corticosteroid, it will block the inflammatory response.
- Eysuvis is manufactured by Alcon laboratories, inc.
How does it work?
- To enhance the efficacy of Eysuvis, Ampplify® Drug Delivery technology is used. It uses mucus-penetrating particles.
- Mucus has the potential to obstruct The delivery of topical ophthalmic drugs. Since the ocular surface is continuously exposed to the surrounding environment, it is necessary for the eyes to have an inbuilt immune mechanism. But this will limit the ability of the drugs to penetrate into the ocular tissue.
- Mucus-penetrating nanoparticles will help in the quick passage of molecules through mucins in the tear film. This prevents adhesion and enables improved distribution and absorption of ocular surface tissue. It specifically targets the cornea and conjunctiva.
- This is why Eysuvis was developed using Ampplify® Drug Delivery 2Overview| Researched based study from Fda.gov technology to promote absorption on the oculus surface. This was achieved by making small molecules (approximately 300 nm), and applying a surface coat.
Uses
Eysuvis uses
- Eysuvis is approved in 2020 for temporary treatment of symptoms of dry eye disease. It must only be used for 2 weeks when you are having dry eye symptoms. Corticosteroid eye drops like Eysuvis are highly recommended by the American Academy of ophthalmology as a treatment for dry eye disease.2Uses| Researched based study from Fda.gov
How to use Eysuvis?
Before instilling every dose of Eysuvis make sure that you:
- Wash your hands thoroughly
- Remove your contact lenses if you are wearing them.
- Shake the solution for 3-4 seconds.
- Do not touch the dropper tip with your fingers or any surface of the eye
- Then pour 1-2 drops in each eye as recommended by the doctor.
Side effects
Eysuvis Side effects
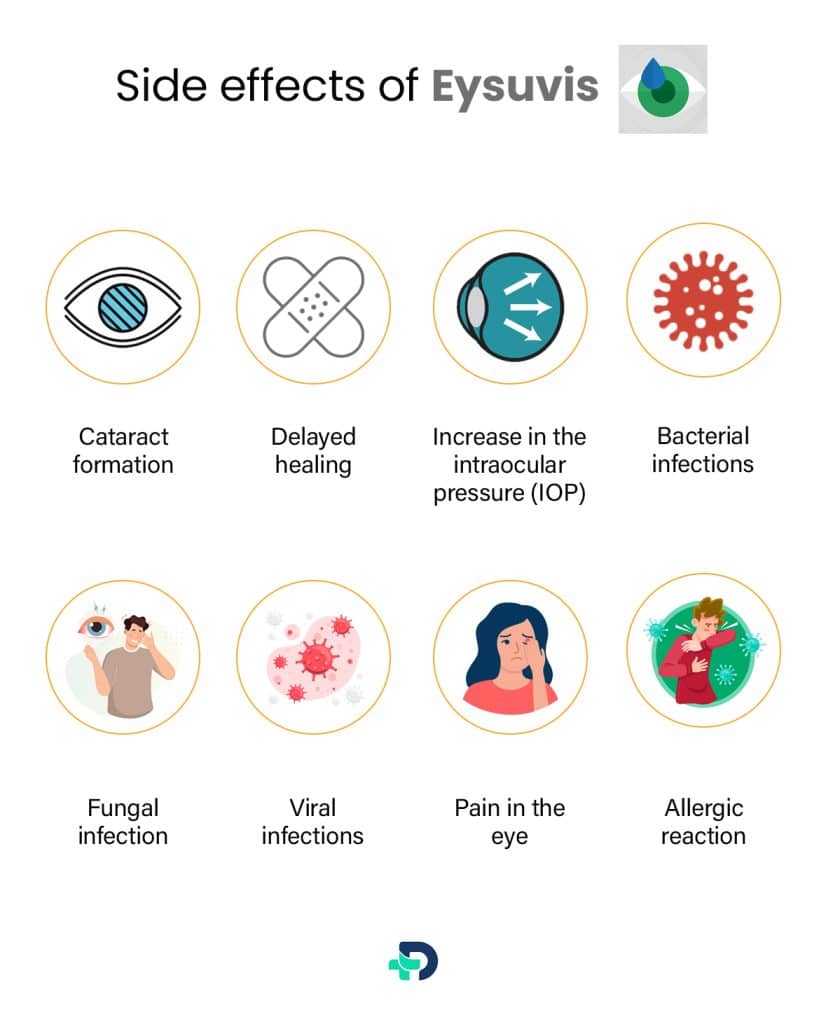
The side effects of Eysuvis are:
- Cataract formation
- Delayed healing
- Increase Intraocular pressure
- Bacterial infections
- Fungal infection
- Viral infections
- Pain in the eye
- Allergic reaction
Cataract formation
- Posterior sub capsular cataract is noticed with the use of ophthalmic corticosteroids.
Delayed healing
- Slow or delayed healing with corneal thinning is noticed with the use of ophthalmic corticosteroids. Application of topical corticosteroids in the thin corneal tissue will lead to corneal perforation.
Increase in the intraocular pressure (IOP)
- Long-term use of corticosteroids will result in damage to the optic nerve, glaucoma and defects in the field of vision and reduced visual acuity. Thus, corticosteroids must be used with precautions if you have glaucoma.
Bacterial infections
- Bacterial infections have the potential to decrease the immune response and elevate the risk of secondary infection. The risk of bacterial infection increases with the use of corticosteroids.
Fungal infection
- Fungal infections that occur in the cornea are highly susceptible with the use of long-term corticosteroid application. If you notice any corneal ulceration, you must suspect fungal invasion due to corticosteroid use.
Viral infections
- If you suffer from herpes Simplex virus, then the use of corticosteroid medications might lead to viral infections. Ocular steroids will not only increase the course of a viral infection,3Side effects| Researched based study from hhs.texas.gov it may also increase the severity of the symptoms.
Pain in the eye
- Ocular pain upon instillation of the drug is the most common side-effect of Eysuvis.
Allergic reaction
- After using Eysuvis, you might notice skin rash, flushing, swelling under the skin, lips, eyelids, tongue, difficulty in breathing, itchiness etc. you must inform your doctor right away if you think you are having an emergency.
Other less common side effects are
- Risk of contamination.
- Optic nerve damage.
- Burning in the eyes.
- Watering of the eyes.
Dosage
Forms and dosage Eysuvis
Available forms are
- Eysuvis is offered in the form of a sterile liquid suspension eye drop. It is available in a concentration of 0.25%. It comes in an 8.3 mL bottle.
- The product is packaged in a white dropper bottle made of low-density polyethylene, with a tip made of linear low-density polyethylene.
- A larger concentration of loteprednol etabonate is absorbed even with less medicine since Eysuvis, made with Ampplify® Drug Delivery technology, claims greater penetrative absorption.
One ml contains
- The active ingredient: loteprednol etabonate 2.5 mg (0.25%)
- The inactive ingredient: glycerin, sodium chloride, sodium citrate dihydrate, Poloxamer 407, citric acid, water for injection and edetate disodium dihydrate.
- The preservative: Benzalkonium chloride 0.01%
Dosage
- The recommended dosage of Eysuvis for the treatment of dry eye is 1-2 drops per eye, administered four times daily. The right number of drops per dose will be informed to you by your doctor. For better results, it is recommended to use this ophthalmic suspension for a maximum duration of two weeks.
What can be done if you miss a dose?
- In case of a missed dose, you must promptly use The ophthalmic solution as soon as you remember. Make sure that you proceed with a regular dosing schedule.
Is long-term use of Eysuvis necessary?
- No, the approved duration for using Eysuvis is restricted to a maximum of two weeks only. If you still noticed dry eye symptoms, even after using it for two weeks, your physician will provide additional treatment courses
Storage
Storage of Eysuvis
- Retain Eysuvis upright (standing position) between 59°F and 77°F (15°C and 25°C).
- Avoid freezing.2Storage| Researched based study from Fda.gov
- Eysuvis can be used until its expiration date after opening. The expiration date is printed on the bottom right side of the bottle label.
- You can throw away the white tamper-evident over cap. To ensure proper preservation, keep the bottle tightly closed and retain the pink cap when the product is not in use.
Precautions
Warnings & Precautions
You must monitor the intraocular pressure and note for any secondary infections.
Consideration with other medications:
- Eysuvis can be prescribed along with other medications like artificial tears that are used for the treatment of dry eye disease.
- To achieve optimal effectiveness and to decrease potential interactions, it is advised to wait for five minutes before you administer Eysuvis after other ocular drops, gels or ointments. The division of doses in this way will promote better absorption of every medication into the eye and it also prevents dilution of medications.
Contact lens
- The active preservatives of Eysuvis have the potential to be absorbed by soft contact lenses. You must remove contact lenses before applying eye drops and wait for 15 minutes before reinserting them.
Pregnancy
- There are no controlled studies noted with loteprednol etabonate in women who are conceiving.
Lactation
- There is no sufficient data on the existence of loteprednol etabonate in human milk, its impact on the infant, or on the milk production.
Pediatric considerations
- Pediatric patients 4Precautions| Researched based study from Sciencedirect.com have not yet been subject to safety and efficacy testing.
Geriatric considerations
- No general changes in safety and efficacy have been identified between older and younger adult patients.
Drug interactions
- Before using Eysuvis, it is essential for you to inform your doctor about the medications that you are taking including vitamins, herbs, or other supplements to avoid any unwanted drug interactions.
Vs Inveltys
Inveltys vs Eysuvis
Inveltys and Eysuvis add prescription medications that are used to treat eye diseases.
Active component
- The active component in Inveltys is loteprednol etabonate, which is a corticosteroid.
- While Eysuvis, on the other hand, has the active ingredient loteprednol etabonate only, but it is formulated by nanoparticle technology.
Uses
- Inveltys is used for the treatment of postoperative swelling and pain after ocular surgery. It is specifically utilized after cataract surgery.
- Eysuvis is indicated for the short-term solution of dry eye disease. It helps in relieving the symptoms of dry eye disease such as eye pain and inflammation.
Dosage
- The recommended dosage for Inveltys is One drop in to the affected eye given for four times every day for a period of two weeks.
- Eysuvis is administered as One drop twice daily for a period of two weeks.
Takeaway
Takeaway
Eysuvis is a highly effective solution for a wide range of patients suffering from dry eye disease. It is the initial prescription given for patients with episodes of dry eye flares as artificial tears. It can also be given in patients who have undergone a surgery, cataract or refractive surgery for the correction of dry eye.
Any feedback on this article?
 This Articles content was accurate
This Articles content was accurate Very Informative Article
Very Informative Article I have a question or a comment
I have a question or a comment
 This article contains inaccurate content
This article contains inaccurate content This article was not helpful
This article was not helpful I have a question or a comment
I have a question or a comment
We appreciate your helpful feedback!
Checkout our social pages
References
-
National Library of Medicine
What’s new in dry eye disease diagnosis? Current advances and challenges | Overview
-
U.S FOOD & DRUG ADMINISTRATION
EYSUVIS-information | Overview | Uses | Storage
-
Texas Health Human Services
Ophthalmics, Anti-Inflammatory/Immunomodulator Therapeutic Class Review (TCR) | Side effects
-
Science Direct
Side effects of drugs used in ocular treatment | Precautions