Entyvio: Uses, Dosage and Side effects

- Entyvio
- 17 Aug 2023
Overview
What is Entyvio?
Entyvio is a brand-name prescription of the drug vedolizumab, a medication primarily used to treat certain autoimmune diseases, specifically inflammatory bowel diseases (IBD) such as ulcerative colitis and Crohn’s disease.
In this article, we will delve into the various aspects of Entyvio, including its mode of action, uses, dosage, administration, side effects, storage, risks, precautions, and interactions with other drugs.

How does it work?
- Entyvio belongs to a class of drugs referred to as integrin receptor antagonists.1Overview| Researched based study from Medlineplus.gov
- It is a unique biological medication that targets and blocks α4β7 integrin, which plays a role in migrating specific white blood cells known as lymphocytes to the gut.
- Thus, preventing its migration to the gut, reducing inflammation, and allowing healing in people with ulcerative colitis and Crohn’s disease2Overview| Researched based study from Entyvio.com
Uses
Uses of Entyvio
Entyvio is an FDA-approved drug used to treat moderate to severe:
- Active ulcerative colitis (UC) in adults.
- Active Crohn’s disease (CD) in adults.
Adult Ulcerative Colitis (UC)
- Entyvio is prescribed to adults with moderate to severe ulcerative colitis (UC) when previous ulcerative colitis drugs are ineffective or not tolerated.3Uses| Researched based study from Fda.gov
- Entyvio effectively achieves symptom remission in people with moderate to severe UC.
Adult Crohn’s Disease (CD)
- Entyvio is prescribed to adults with moderate to severe Crohn’s disease (CD) when previous ulcerative colitis drugs are ineffective or not tolerated.4Uses| Researched based study from Fda.gov
- Entyvio effectively achieves symptom remission in people with moderate to severe CD.
Dosage
Dosage and administration
A healthcare professional administers Entyvio as an infusion through vein (IV). The recommended dosage for both ulcerative colitis and Crohn’s disease is:
- 300 mg, administered as a thirty-minute IV infusion at zero, two, and six weeks following the initial dose.5Dosage| Researched based study from Entyviohcp.com
- Subsequently, maintenance doses are administered every eight weeks after that.
- The infusion rate should be reduced or discontinued if any negative reactions show up.
Storage
Storage of Entyvio
- Entyvio should be stored in a refrigerator at 2°C to 8°C (36°F to 46°F).
- Before administration, the medication should be brought to room temperature.
- For 15 seconds, gently swirl the container so it dissolves the lyophilized powder.
- Do not forcefully shake or invert the container.
Side effects
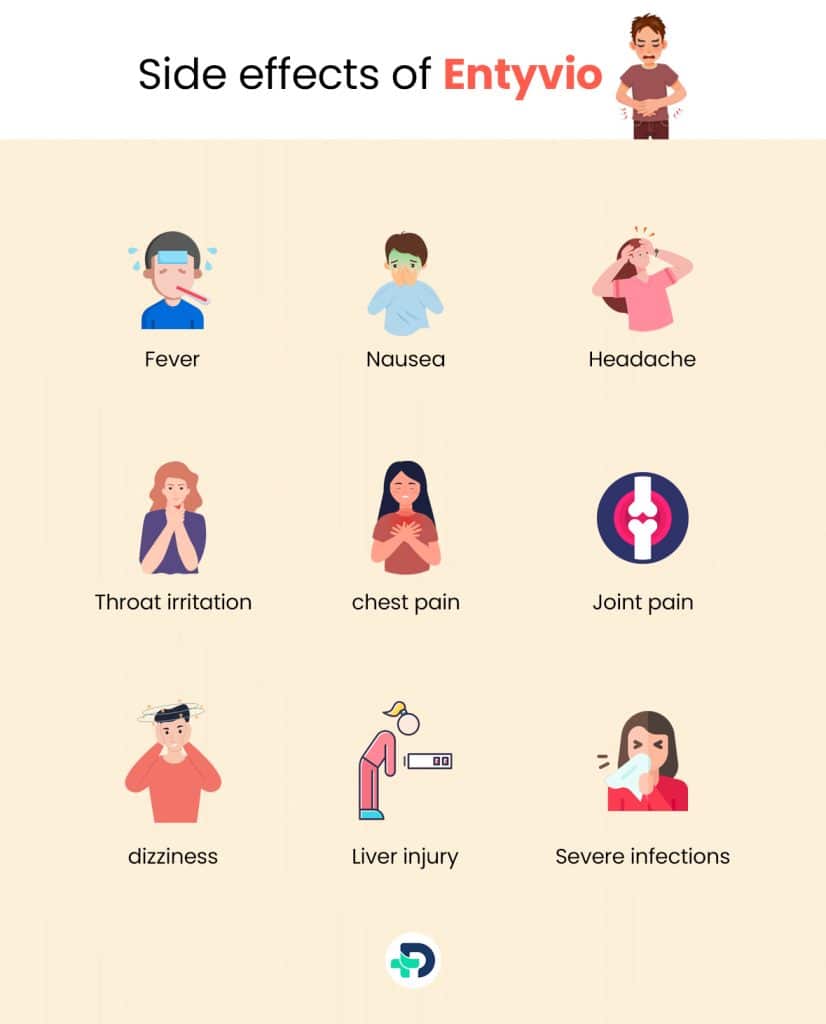
Side effects of Entyvio
The most common side effects of Entyvio are generally mild to moderate in severity and may include:
- Fever
- Headache
- Nausea
- Joint pain
- Throat irritation
These mild symptoms may go away without any treatment. However, it is critical to tell a healthcare expert if any of these symptoms worsen or prolong.
Some severe side effects may include:
- Severe infections – fever, cough, red or painful skin sores on the body, chills, sore throat, pain during urination, or sinusitis symptoms.
- Infusion-related reactions – shortness of breath, lightheadedness, or chest pain.
- Liver injury – loss of appetite, fatigue, right upper belly pain, dark urine, nausea, yellowing of skin or eyes, light-colored stools.6Side effects| Researched based study from Clevelandclinic.org
- Hypersensitivity reactions – like trouble breathing, skin rashes, swelling of the lips, face, tongue, or throat, hives, itching, trouble swallowing, increased heart rate.
- Progressive Multifocal Leukoencephalopathy (PML) – dizziness, confusion, loss of balance, problems thinking, change in how people walk or talk, weakness on one side of the body, blurred vision, and loss of vision.
Potential Risks
Risks of Entyvio
While Entyvio can be highly effective in treating certain autoimmune conditions, it is essential to consider potential risks associated with its use :
- Entyvio can cause severe hypersensitivity reactions in some individuals, and people with a history of allergic reactions to vedolizumab or any of its components should not be given Entyvio.
- Entyvio increases an individual’s risk of infections, and patients need to inform their healthcare provider about any history of infections, including tuberculosis or hepatitis B or C.
- This drug can also injure the liver, and patients with any history of liver problems, alcohol misuse, or hepatitis infection should inform the doctor.
- Even though the risk of developing progressive multifocal leukoencephalopathy (PML) is low, it is still possible, and patients should be informed to report any of its symptoms during treatment.
- Some patients can develop infusion reactions during or after an IV infusion of Entyvio. If a severe infusion reaction occurs, the treatment should be discontinued promptly and proper medical attention sought.
Precautions
Precautions
- People with a known history of allergy to Entyvio, vedolizumab, or any of its components should not be given Entyvio.
- Before starting Entyvio, discussing the patient’s medical history and current medical conditions with their healthcare provider is crucial.
- Patients taking Entyvio should be monitored closely for signs of infection, such as cough, fever, or other flu-like symptoms.
- Women who think they are pregnant or plan to get pregnant before or while taking Entyvio should inform their doctor.
- Breastfeeding mothers should talk to their healthcare providers before using Entyvio, as there are no adequate studies on women to determine any risk to infant while using this medication.7Precautions| Researched based study from Mayoclinic.org
- It is important to note that Entyvio is not recommended for use in pediatric patients, as its safety and efficacy have not been established in this population.
- While on treatment with vedolizumab, people should not have any vaccines without their doctor’s approval. Before receiving vedolizumab, all vaccines must be complete.7Precautions| Researched based study from Mayoclinic.org
Interactions
Interactions of Entyvio
Certain medicines and substances when taken with Entyvio, potentially reduces its efficacy or increases the likelihood of developing negative effects. These may include:
- TNF blockers – should not be used concurrently with Entyvio to avoid the possibility of an increased risk of infections.8Interactions| Researched based study from Fda.gov
- Natalizumab – should not be used concurrently with Entyvio to avoid the potential for increased risk of PML and other infections.8Interactions| Researched based study from Fda.gov
- Live vaccines – like that given for Measles, mumps, rubella (MMR), chickenpox, rotavirus, etc. should be avoided while on Entyvio therapy due to the immunosuppressive effects of the medication.8Interactions| Researched based study from Fda.gov
- Alcohol – Consuming alcohol may not interact with the Entyvio but may worsen some side effects like nausea, vomiting, and headache and may also increase the risk of liver injury.
Before starting Entyvio, it is recommended that people discuss all the medications they are currently taking, including prescription drugs, over-the-counter medicines, herbal drugs, and supplements, as the above list of drug interactions does not cover all the medications known to interact with Entyvio. They will give customized guidance depending on an individual’s condition and medical requirements.
Bottom Line
The Bottom Line
When administered as directed, Entyvio can effectively treat people with ulcerative colitis or Crohn’s disease who experienced an insufficient response to prior medications. However, it is accompanied by possibility of developing adverse effects ranging from moderate to severe, as with any medicine. Close monitoring and communication with healthcare providers are essential to manage and address adverse reactions. Always consult a healthcare provider for specific information and recommendations regarding Entyvio or other medications.
Any feedback on this article?
 This Articles content was accurate
This Articles content was accurate Very Informative Article
Very Informative Article I have a question or a comment
I have a question or a comment
 This article contains inaccurate content
This article contains inaccurate content This article was not helpful
This article was not helpful I have a question or a comment
I have a question or a comment
We appreciate your helpful feedback!
Checkout our social pages
References
-
Medline Plus
Vedolizumab Injection | Overview
-
Takeda Pharmaceuticals U.S.A
ENTYVIO | Overview
-
U.S FOOD & DRUG ADMINISTRATION
Drug Trials Snapshot: Entyvio (vedolizumab) to Treat Ulcerative Colitis | Uses
-
U.S FOOD & DRUG ADMINISTRATION
Drug Trials Snapshot: Entyvio (vedolizumab) to Treat Crohn's Disease | Uses
-
Takeda Pharmaceuticals U.S.A
ENTYVIO | Dosage
-
Cleveland Clinic
Vedolizumab Injection | Side effects
-
Mayo Clinic
Drugs and Supplements-Vedolizumab (Intravenous Route) | Precautions
-
U.S FOOD & DRUG ADMINISTRATION
ENTYVIO (vedolizumab) for injection | Interactions